Figure 1.
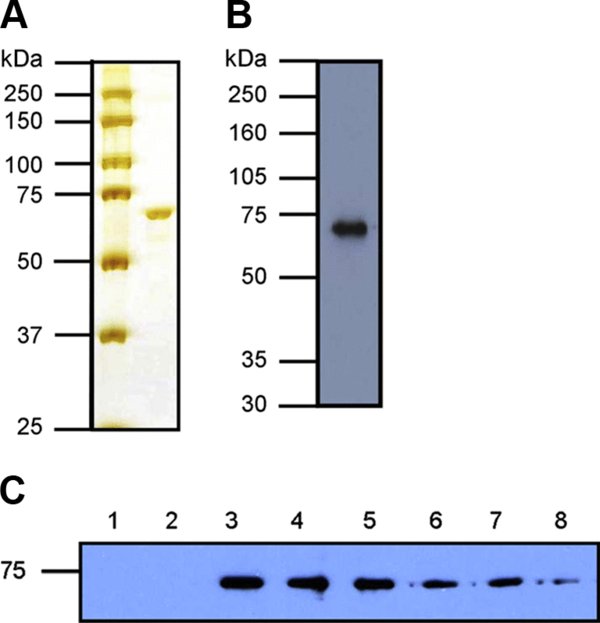
Purification of recombinant bovine Hsp70 and formation of Hsp70:peptide complexes. (A) Purified Hsp70 (1 μg) was resolved by SDS-PAGE on a 10% gel and detected by silver staining. Left hand lane indicates molecular weight markers. (B) Purified Hsp70 (250 ng) was analysed by Western blot using an anti-Hsp70 monoclonal antibody (SPA-810, Stressgen). (C) 2 μM Hsp70 was incubated with 60 μM biotinylated peptide and increasing amounts of unlabelled peptide in 55 μL PBS at 37 °C for 1 h to form complexes. Fractions containing an equivalent of 1 μg Hsp70 were resolved by non-reducing SDS-PAGE on a 10% gel, followed by Western blot analysis using streptavidin-HRP to detect biotinylated peptide. Controls of Hsp70 and biotinylated peptide only were run in lanes 1 and 2 respectively. Lanes 4–8 additionally contain unlabelled peptide at 6 μM, 60 μM, 150 μM, 300 μM and 600 μM (0.1×, 1×, 2.5×, 5× and 10× molar concentration of labelled peptide). (A color version of this figure is available at www.vetres.org.)


