Figure 1.
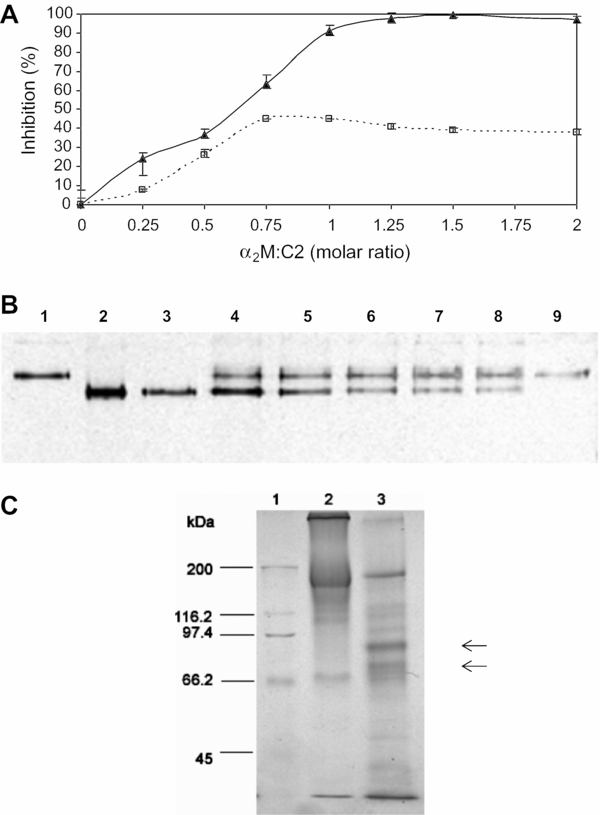
The interaction of the recombinant catalytic domain of congopain (C2) with bovine α2M. (A) The effect of increasing amounts of α2M on the activity of C2 against hide powder azure (▲) and Bz-Pro-Phe-Arg-pNA (□). Different concentrations of α2M were incubated with C2 (100 pmol for hide powder azure assay; 20 pmol for Bz-Pro-Phe-Arg-pNA assay) at molar ratios of 0.25:1, 0.5:1, 0.75:1, 1:1, 1.25:1, 1.5:1 and 2:1 for 20 min at 37 °C. Proteolytic activity was determined by the extent of hydrolysis of substrate compared to that of a C2 control with no α2M (100% activity), and expressed as a percentage inhibition. Error bars represent the ± SEM (n = 3). (B) Non-denaturing PAGE (5% gel) analysis of the interaction between bovine α2M and activated C2. Lanes 1 and 9 500 ng α2M; lanes 2–8, α2M (500 ng) was incubated with increasing amounts of C2 (11–91 ng), corresponding to molar ratios of α2M:C2 of 0.25:1, 0.5:1, 0.75:1, 1:1, 1.25:1, 1.5:1 and 2:1 in the respective lanes. Proteins were silver stained. (C) Reducing SDS-PAGE (7.5% gel) after reaction of bovine α2M with activated C2. Lane 1, Bio-Rad molecular weight markers consisting of myosin (200 kDa), β-galactosidase (116.2 kDa), phosphorylase b (97.4 kDa), bovine serum albumin (66.2 kDa), and ovalbumin (45 kDa); lane 2, α2M (10 μg); lane 3, C2-α2M complex (10.372 μg). Proteins were stained with Coomassie blue R-250. Arrows represent bands at 95 and 85 kDa.


